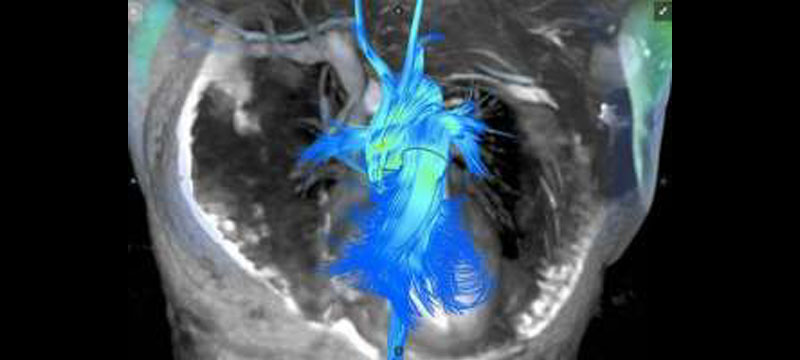
Arterys, a pioneer in cloud-based medical imaging software, has received 510(k) clearance from the US Food and Drug Administration (FDA) to market its Arterys Cardio DLTM application. Arterys Cardio DLTM is the first technology to be cleared by the FDA that leverages cloud computing and deep learning in a clinical setting. Arterys Cardio DLTM provides automated, editable ventricle segmentations based on conventional cardiac MRI images that are as accurate as segmentations performed manually by experienced physicians. The US clearance complements…