A newly developed microscope is providing scientists with a greatly enhanced tool to study how neurological disorders such as epilepsy and Alzheimer’s disease affect neuron communication. The microscope is optimized to perform studies using optogenetic techniques, a relatively new technology that uses light to control and image neurons genetically modified with light-sensitive proteins.
“Our new microscope can be used to explore the effects of different genetic mutations on neuronal function,” said Adam Cohen from Harvard University, USA, and the leader of the research team that developed the microscope. “One day it could be used to test the effects of candidate drugs on neurons derived from people with nervous system disorders to try to identify medicines to treat diseases that do not have adequate treatments right now.”
The new microscope, called Firefly, can image a 6-millimeter-diameter area, more than one hundred times larger than the field of view of most microscopes used for optogenetics. Rather than studying the electrical activity of one neuron, the large imaging area makes it possible to trigger the electrical pulses neurons use to communicate and then watch those pulses travel from cell to cell throughout a large neural circuit containing hundreds of cells. In the brain, each neuron typically connects to one thousand other neurons, so viewing the larger network is important to understanding how neurological diseases affect neuronal communication.
In The Optical Society (OSA) journal Biomedical Optics Express, Cohen and his colleagues report how they assembled the new microscope for less than $100,000 using components that are almost all commercially available. The microscope not only images a large area, but also collects light extremely efficiently. This provides the high image quality and fast speed necessary to watch neuronal electrical pulses that each last only one thousandth of a second.
Using light to see neurons fire
The new microscope is ideal for studying human neurons grown in the laboratory. In the past decade, scientists have developed human cell models for many nervous system disorders. These cells can be genetically modified to contain light-sensitive proteins that allow scientists to use light to make neurons fire or to control variables such as neurotransmitter levels or protein aggregation. Other light-sensitive fluorescent proteins turn the invisible electrical pulses coming from neurons into brief flashes of fluorescence that can be imaged and measured.
After stimulating the neurons, the microscope uses a camera imaging at a thousand frames a second to capture the fluorescence induced by the extremely short electrical pulses. “The optical system must be highly efficient to detect good signals within a millisecond,” said Cohen. “A great deal of engineering went into developing optics that can not only image a large area but do so with very high light collection efficiency.”
Watching 85 neurons at once
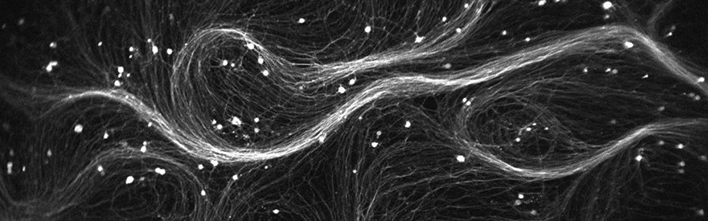
The researchers demonstrated their new microscope by using it to optically stimulate and record the fluorescence from cultured human neurons. “The neurons were a big tangled mess of spaghetti,” said Cohen. “We showed that it was possible to resolve 85 individual neurons at the same time in a measurement that took about 30 seconds.”
After the initial stimulation and imaging, the researchers were able to find 79 of those 85 cells a second time. This capability is important for studies that require each cell to be imaged before and after exposure to a drug, for example.
In a second demonstration, the researchers used the microscope to map the electrical waves propagating through cultured heart cells. This showed that the microscope could be used to study abnormal heart rhythms, which occur when the electrical signals that coordinate heartbeats do not work properly.