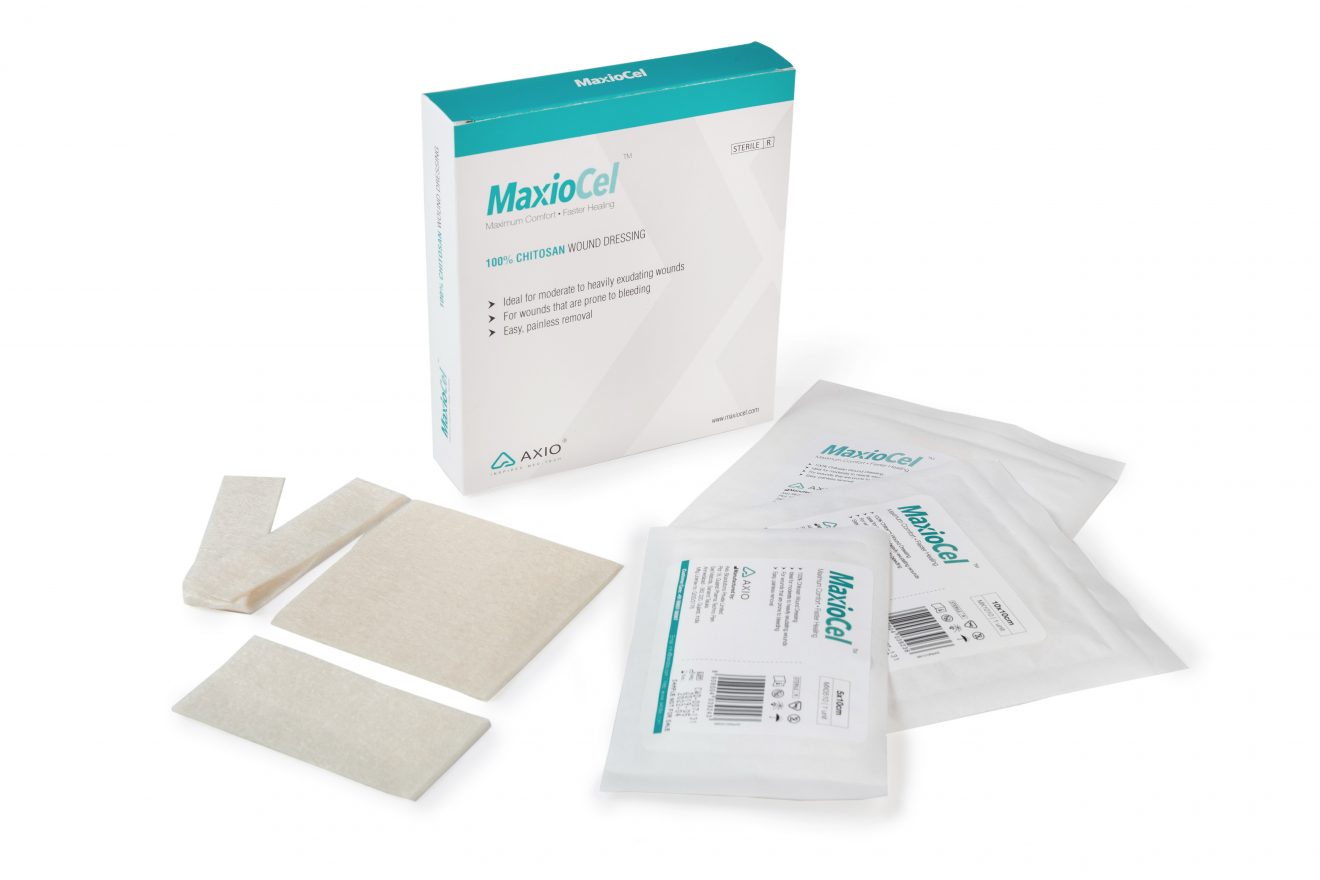
Integrated woundcare company, Axio Biosolutions recently announced that it has received European CE Certification from DNV GL Norway for its next generation advanced wound care product range, MaxioCel. The regulatory approval paves the path for Axio to tap into a fast growing $15 billion advanced woundcare market, globally.
This next-generation advanced woundcare dressing, MaxioCel is based on Bioactive Microfiber Gelling technology that accelerates healing through quicker granulation. It also provides comfort and has shown faster healing on chronic wounds, such as pressure ulcers, diabetic foot ulcers, venous leg ulcers, cavity wounds, skin abrasions, donor sites and post-operative surgical wounds during clinical studies.
Commenting on MaxioCel from one of the centers in Europe , Dr. Christophe Marchand, Vascular Surgeon, Head of Center for Woundcare at Clinique Inkerman , Niort, France said, “MaxioCel provides a complete response in the management of complex chronic wounds: bacteriostatic, fibrinolytic, hemostatic, exudate absorption and easy, atraumatic removal facilitating patient adhesion to treatment.”
With more than 100,000 units shipped since its launch 18 months ago, MaxioCel has had a huge impact already in Asian market. Patients are being treated with MaxioCel in 100+ hospitals in South Asia and it has shown to have significant improvement in healing time and complete closure of wounds with minimal scars. A highly active advanced wound dressing that effectively manages exudates, pain relief, and scar improvement for moderate to heavily exudating wounds, MaxioCel has become a preferred product among top vascular and endovascular surgeons and plastic surgeons within a short period.
Dr. Raghuram Sekhar, Consultant Vascular and Endovascular Surgeon, Kokilaben Dhirubhai Ambani Hospital, Mumbai, India who has been a long term user of Maxiocel affirms, “MaxioCel is a unique and advanced wound care dressing material. In different kind of cases that we used it, we witnessed rapid granulation and overall wound improvement while keeping the patients comfortable.”
The CE approval will now help Axio with access to the second largest global market for advanced woundcare products. As part of its strategic plans, Axio will soon be launching MaxioCel in 15+ countries in Europe including reimbursement markets with multi-million revenues in this year itself. Present in over 28 countries, Axio is among the very few medtech startups from India that has successfully ramped up its global presence with its unique, innovative and disruptive life-saving products.
Responding to the CE certification and subsequent access to the European market, Leo Mavely, Founder and CEO, Axio Biosolutions said, “At Axio, our innovations are designed looking at how to provide best-in-class treatment to patients at most affordable cost. Be it Axiostat, which became the only Indian hemostat to receive an approval from USFDA or MaxioCel, which has now been certified by CE for its effectiveness, each of our product milestones reiterate our commitment to this vision. Since its launch, MaxioCel has made a huge impact on patient lives and the medical fraternity . We are excited to have the opportunity to extend this innovative and highly effective solution into global markets.”
Globally, it is estimated that one to two per cent of the population in developed countries will experience a chronic wound during their lifetime. This has a significant impact on health and healthcare systems, with countries like US and UK spending close to USD 5 billion per year on chronic wound-related implications. Apart from factors like age, the growing prevalence of diabetes, which is expected to affect 600 million people by 2030 further contribute to the number of patients who require chronic wound care management. Advanced woundcare market is seen as next frontier in medtech and has seen a lot of consolidation in last few years. 3M Healthcare had recently acquired Acelity (KCI+Sytagenix) for $6.7 Billion.
In line with its vision to provide world-class wound care to every patient in a timely manner, despite the challenges posed by the COVID-19 pandemic, Axio made the strategic decision to venture into the retail space in India in 2020.