Abbott has announced that its Navitor™ transcatheter aortic valve implantation (TAVI) system has received CE Mark in Europe for an expanded indication. The approval allows the device to treat people with symptomatic, severe aortic stenosis who are at low or intermediate risk for open-heart surgery. Previously, in 2021, Navitor received CE Mark for patients at high or extreme surgical risk. With this new clearance, the system is now available for patients across all surgical risk categories in Europe, significantly broadening access to treatment for a larger population.
The expanded indication is supported by positive safety and efficacy results from the VANTAGE study, presented as a late-breaking trial at the European Society of Cardiology (ESC) Congress 2025 in Madrid (Aug. 29–Sept. 1, 2025). These data were simultaneously published in JACC: Cardiovascular Interventions.
“The VANTAGE study provides the scientific backbone for expanding Navitor’s indication to low- and intermediate-risk patients. The data are exceptional across both populations, confirming that the Navitor valve performs precisely as designed,” said Nicolas van Mieghem, M.D., medical director of the department of interventional cardiology at the Thoraxcenter, Erasmus University Medical Centre, in the Netherlands, who serves as principal investigator of the VANTAGE trial. “Up to 50% of younger patients with aortic stenosis will also get coronary artery disease in later years, and Navitor’s design preserves options and ability for lifetime disease management if future cardiac interventions are required.”
Aortic stenosis occurs when the aortic valve’s opening narrows, restricting blood flow to the body. Left untreated, it can lead to heart failure and death. The Navitor TAVI device replaces the aortic valve through a minimally invasive procedure and is delivered to the heart through a small incision in the leg. The performance of such devices is measured by blood flow through the valve, referred to as hemodynamics.
Key findings from the VANTAGE trial
The late-breaking data presented at ESC from Abbott’s VANTAGE study showed Navitor met all safety and effectiveness primary endpoints, supporting its expansion to treat people with symptomatic, severe aortic stenosis who are at low or intermediate surgical risk. Key findings include:
- Excellent safety. In the first 262 patients with 12-month follow-up completed, there was a low rate (2.3%) of all-cause mortality or fatal stroke/stroke with disability.
- Proven effectiveness. No patients at 30 days had moderate or greater PVL (paravalvular leak or backflow of blood around the valve) and only 13.6% had mild PVL, a rate that is considered low.1,2,3,4
- High technical success: There was a high rate of technical success (97%) with no procedural deaths.
- Sustained hemodynamic performance. Excellent hemodynamic performance was seen at 12 months.
“Navitor is a strong example of how Abbott continues to evolve its structural heart portfolio to meet the growing demand for minimally invasive alternatives to open-heart surgery,” said Sandra Lesenfants, senior vice president of Abbott’s structural heart business. “Aortic stenosis is a life-threatening condition that can progress rapidly, and this expanded indication for Navitor means that patients have more options that can help reduce their symptoms and improve their lives.”
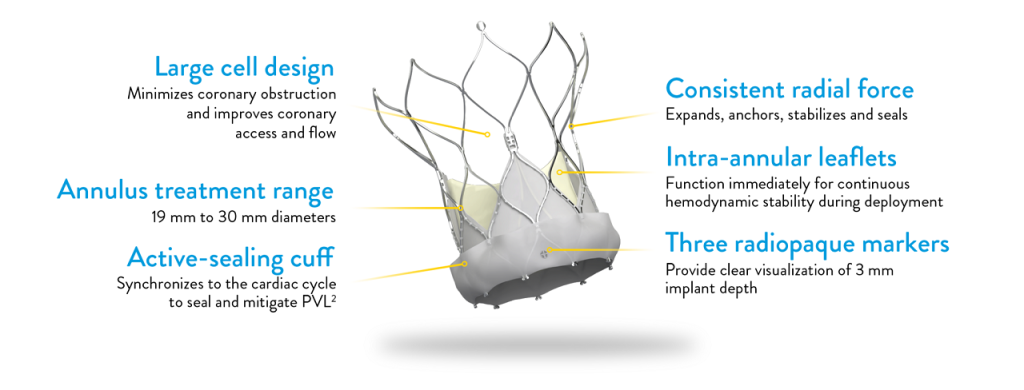
The Navitor TAVI system is currently approved in the U.S. to treat people with symptomatic, severe aortic stenosis who are at high or extreme risk for open-heart surgery.
Updated transcatheter edge-to-edge repair (TEER) guidelines
During ESC Congress 2025, ESC and the European Association for Cardio-Thoracic Surgery (EACTS) announced new guidelines for the management of valvular heart disease. Mitral valve TEER was upgraded from a treatment that should be considered (IIa) to a recommended treatment (Class Ia) for selected patients with severe functional (or secondary) mitral regurgitation (MR). Tricuspid valve TEER was also upgraded from a treatment that may be considered (IIb) to a treatment that should be considered (Class IIa) for selected patients with severe functional tricuspid regurgitation (TR).
Also Read: Dr. Suhail AlRukn: Championing Stroke Awareness and Treatment
With these updated guidelines, there’s additional support for the use of MitraClip™ and TriClip™ for MR and TR patients that is backed by evidence from multiple clinical studies, including COAPT, TRILUMINATE, TRILUMINATE Pivotal, bRIGHT, RESHAPE-HF2 and TRI.fr, that demonstrate the therapies’ effectiveness.