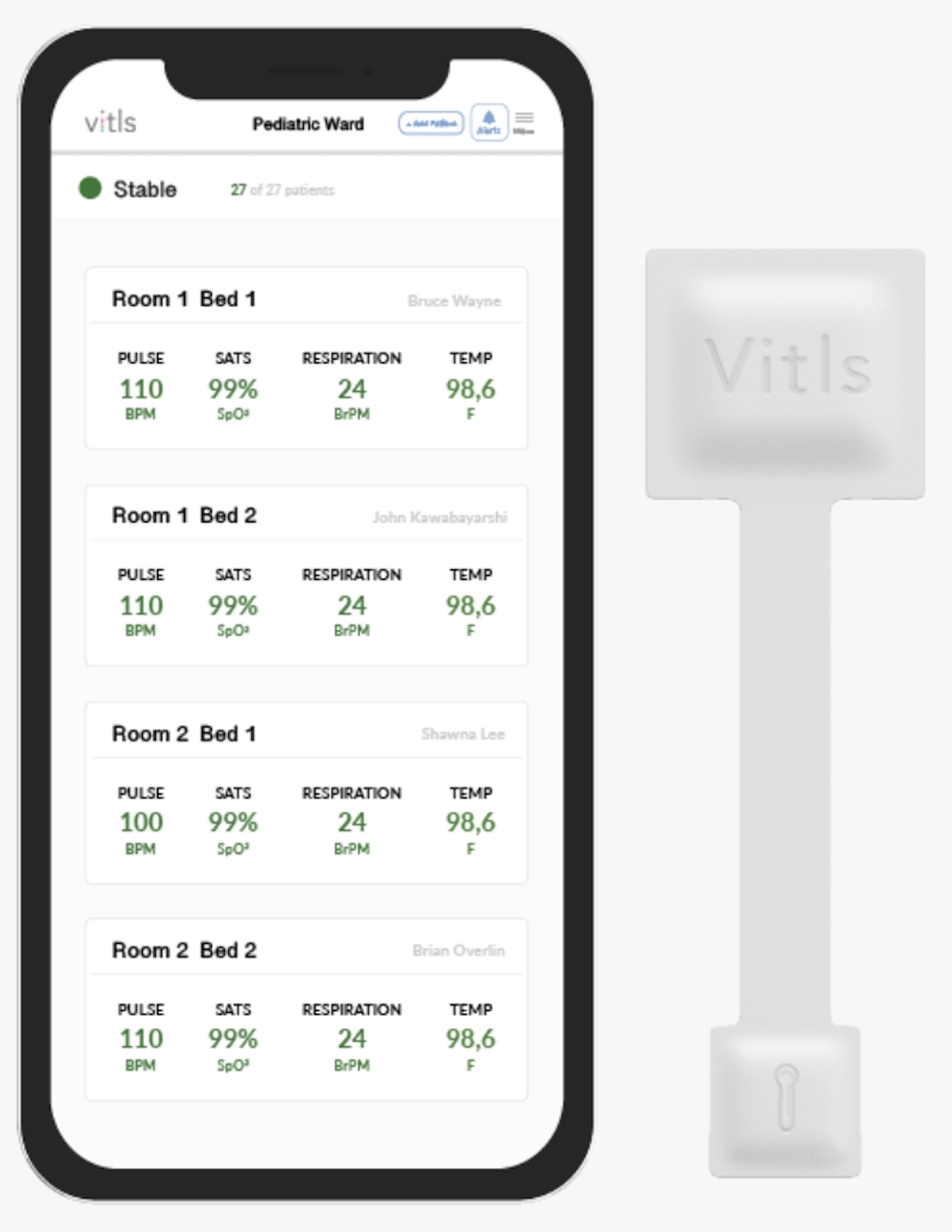
Vitls Inc., a company out of Houston, Texas, won FDA clearance for its Vitls Platform, a combination of a vitals monitoring patch, cloud storage, and an app that lets clinicians monitor multiple patients wearing such a patch at once.
The patch, which is called Tégo, keeps track of a patient’s heart and respiration rates, heart rate variability, body temperature, and blood oxygenation (SpO2). It’s a wireless, flexible, disposable device with a battery lifetime of about six days. Patients can be sent home wearing one while still being closely monitored remotely by their clinical team.
“When my eldest son suffered from febrile seizures, we couldn’t find anything reliable and accurate enough to alert us at the onset of his fevers; we developed Tégo to address the challenge of intermittent monitoring and late detection for both patients and clinicians,” said Werner Vorster, chief executive officer and founder of Vitls Inc., in a press release. “With this FDA clearance and our US launch planned for Q3/2020, we are much closer to our goal of making continuous monitoring the standard of care in healthcare settings, as well as in the home. Healthcare needs accurate, clinical grade, continuous monitoring – especially now during COVID-19 – and we’re proud and fortunate to be in a position to help.”
The Vitls app can be used to program instant notifications for every patient, to see trends over the several days that the patient was monitored, and record notes that can be synced with the clinic’s electronic health record (EHR). The Vitls Platform is compatible with iOS and Android devices, as well as most browsers and many EHRs.