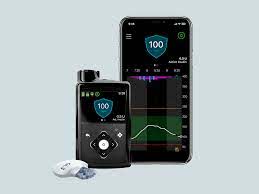
Medtronic won the EU CE Mark for its MiniMed 780G closed loop insulin pump that features both Medtronic’s own SmartGuard algorithm and MD-Logic, an algorithm developed by DreaMed Diabetes, a small Israeli firm. The system, indicated to be used by patients with type 1 diabetes between ages 7 and 80, automatically delivers both basal insulin and correcting boluses every five minutes, if necessary.
Such close monitoring and control of blood glucose levels may help patients stay within a healthy range while reducing worry about forgetting to track every instance of food intake. Target glucose levels can be set to as low as 100 mg/dL (5.5 mmol/L), which is lower than that of other advanced closed-loop insulin pump systems. Thanks to Bluetooth wireless connectivity, the system can raise alarms and share its readings with the patient’s smartphone, as well as those of caretakers and physicians.
Europeans living with Type 1 diabetes will have a chance to get a miniMed 780G this Fall, while Americans will probably have to wait longer as the system is still considered an investigational device and not approved for sale in the United States.