“Built on Nallian’s data sharing platform, the Global Pharma Tracker integrates logistics, temperature and quality data into a single, real-time view of a shipment’s journey, from factory to pharmacy. Data is injected in the data sharing platform from the responsible parties’ systems, leveraging legacy systems and previous IT investments,” says Verheyen
 In order for medications, vaccines, and other pharmaceuticals to remain effective, they require optimal conditions in which they are stored in, cared for, and monitored.
In order for medications, vaccines, and other pharmaceuticals to remain effective, they require optimal conditions in which they are stored in, cared for, and monitored.
The environment inside pharmaceutical locations should be constantly observed. The slightest shih in temperature may consequently change the potency and sterilization.
Preserving the quality of pharmaceuticals highly depends significantly on how it is being maintained, transported and stored along the cold chain. In its simplest form, the product ships directly from manufacturer to end user or customer. In reality, however, the chain is rarely this short.
Proper storage temperatures are the basic requirements to ensure optimum quality of the pharmaceuticals throughout their shelf life. Increasingly, drugs and vaccines should be stored and transported within a specific temperature range: between 2 ̊ and 8 ,̊ or between 20 ̊and 25 .̊
In particular, vaccines, insulin, and biotechnology-derived products must be protected from freezing. Even a brief period at sub-zero temperatures may cause an irreversible loss of efficacy. These products should be maintained
within a narrow temperature range above freezing but below maximum temperature.
Jean Verheyen, CEO of Nallian tells Mediworldme how their Global Pharma Tracker provides unparalleled levels of transparency and visibility to the whole pharma supply chain.
Tell us in detail about your pharma tracker?
The Global Pharma Tracker is the first independent data sharing platform for end-to-end tracking and monitoring of temperature-controlled pharma shipments. Providing unparalleled levels of transparency and visibility, it empowers pharma actors to efficiently detect, act upon and ultimately prevent costly temperature excursions – currently causing billions worth of product loss every year.
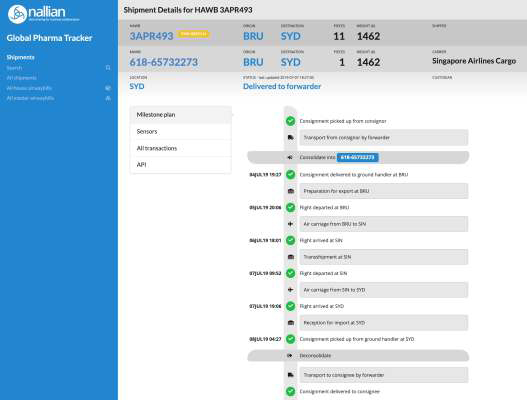 Why is this relevant?
Why is this relevant?
Managing a temperature-controlled supply chain is a challenge. $35 billion worth of pharmaceutical products is lost per annum due to cold chain issues.
•43% of this cost is due to compromised product
•24 % of cost is related to root cause analysis
•It takes in general 3 weeks or more to collect relevant data
How does it end to end track and monitor temperature control pharma shipment?
Built on Nallian’s data sharing platform, the Global Pharma Tracker integrates logistics, temperature and quality data into a single, real-time view of a shipment’s journey, from factory to pharmacy.
It registers all events from the different actors involved in the process (shippers, handlers, carriers, forwarders, cold chain solution providers. Data is injected in the data sharing platform from the responsible parties’ systems, leveraging legacy systems and previous IT investments.
The data owner always stays in control of who sees which part of their data. Only parties related to a shipment can see the data and only those parts of the data that are shared with them in that specific context.
GPT creates a unique overlay of temperature, quality and logistics information (MAWB, HAWB, IoT), providing the visibility needed to make the right decisions in case of excursions or delays, and guarantee a better quality of logistics service to shippers and ultimately, patients.
How does it empower pharma experts to efficiently detect, act upon & ultimately prevent costly temperature excursions – currently causing billions worth of product loss every year?
The overlay of temperature, quality and logistics information as well as registration of the responsible party in the platform provides visibility on when an excursion occurs (or is likely to occur soon), where the shipment is at that time and who is responsible for it. Without GPT, collecting the relevant data to analyze an excursion can take 3 weeks or more. Using GPT, this information in available to all relevant parties. In addition, alerts can be sent in real-time when an excursion or delay occurs.
Analysis of this information also allows to better understand ‘danger zones’ and take pro-active measures. For example: if data analysis shows that at a specific location, excursions happen frequently during a specific period of the day, and this is linked to ambient temperature typically being elevated, one might decide to apply another type of packaging that ensures thermal protection for a longer period of time to ensure the product is better protected while waiting to be loaded; or one might decide to ship the product via another route.
The global pharma air cargo supply chain involves many partners: shippers, carriers, ground handlers, forwarders, cold chain service providers and airport authorities. In this fragmented supply chain, detecting excursions and defining why and where they happened is today a complex, time- consuming and manual process? Where does pharma tracker come in?
The different actors involved in the process may optimize their processes internally, this optimization stops at the company’s boarder – or the handover of the shipment. Data captured during their part of the process remains within their own systems and is not necessarily shared with other parties. The GPT brakes down these siloes by enabling virtual integration of the parties involved. It gathers all relevant data in the data sharing platform and makes it visible to the relevant parties within a clear and predefined data governance and sharing framework.
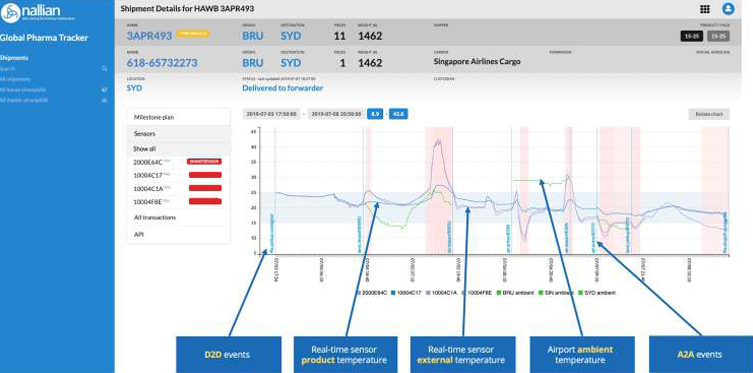
Pharma interestingly is one of the sectors that is most advanced in the when it comes to integrating technology? What do you think?
The pharmaceutical industry is highly regulated and faces increasingly strong requirements in terms of traceability and visibility. This requires it to be forward thinking and leverage technology as an enabler (think of the implementation of serialization in view of the FMD). Within the steering groups of organizations such as Pharma.Aero we see a clear demand and willingness to adopt technology such as GPT – as in many other industries it will be the innovative, forward thinking players who will take the lead and accelerate adoption for the industry.
Keeping in view the t remendous competitive pressure that the pharma industry is facing, efficient management of business processes is becoming essential for sustenance and growth. This is where the pharma industry can leverage technologies that can offer a powerful solution for the pharmaceutical industry and that enables them to be standard compliant and optimize their ROI? Your opinion?
With $35 billion worth of pharmaceutical products lost per annum due to cold chain issues (43% of cost due to compromised product, 24% of cost related to root cause analysis), it goes without saying there are huge benefits to be reaped in terms of ROI. Solutions like the GPT are specifically designed to help pharmaceutical companies avoid these losses, by providing better insights that empower them to take corrective actions faster & take preventive measures that eventually avoid them from happening.
What should Pharma organizations do to achieve digital transformation?
As it is ohen repeated at the leading conferences in the industry, the technology is there, it’s a matter of starting to adopt it. Those parties willing to move forward should not wait for the entire industry to move forward as a whole, but start with a ‘coalition of the willing’. In particular in view of establishing efficient collaboration through data sharing, we have already seen a lot of value in starting with such a small group of people/companies who wish to move forward. It allows booking first successes rapidly, as it required less people to align. It is also how we have successfully run the pilot of GTP (with a limited number of shippers, handlers, forwarder and airports, one or two lanes to track) and it is how other cargo communities have successfully adopted collaborative work (such as Brussels Airport, Heathrow, Vienna Int’l Airport, Liege Airport). The successes these pioneers are booking not only strengthens their leading position in the market but also accelerate adoption in the industry
What does 2020 hold for Pharma industry in terms of technology?
The technology is ready to break the status quo and change the outlook of the industry. Powerful trackers are available to ensure reliable registration throughout the shipments journey, packaging is getting ever more advanced and the GPT platform can provide the visibility and transparency needed. It’s now a matter of adopting it.











