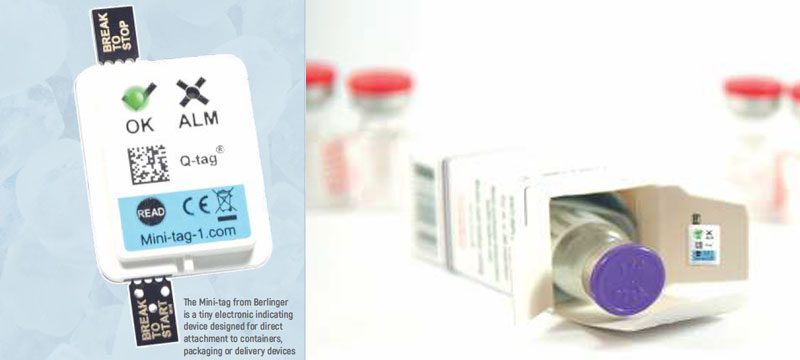
Final mile transportation and subsequent user storage conditions present unique challenges when it comes to ensuring the safe and effective use of pharmaceutical products.
by Corneliu Tobescu, Chief Operation Officer at Berlinger & Co AG
INTRODUCTION
Cold Chain logistics is a $10 billion component of the $1 trillion global pharma industry. Around 80 per cent of drugs require temperature controlled transportation and the trend is distinctly upwards. In some drug categories the proportion is even higher. For example, according to the World Health Organisation, more than 90 per cent of all vaccines require a temperature-controlled supply chain.
The need for these controls is driven by the increasing sensitivity of modern drugs, particularly those of a ‘largemolecule’ i.e. biologic nature. It is a requirement that is underpinned by tight regulatory controls and compounded by factors such as globalised production, new market development and new systems of transportation. And the problem of maintaining the correct product temperature during shipment is not insignificant. According to industry sources the incidence of temperature excursions during pharma transportation may be as high as 5 per cent of the total.
MARKET TRENDS
Currently one of the biggest market trends in pharmaceuticals is the move towards biologic medicines and personalised, patient-centric remedies. The complex nature of biologics and vaccines means that they are often extremely reliant on the maintenance of a highly controlled environment in order to be fully effective or even be viable at all. With the basis for many of these treatments being living cells or complex chemical molecules there is significant risk of therapeutic loss or impairment in the event of unacceptable temperature exposures.
It’s not just the drugs themselves that are becoming more complex. The supply chains involved in moving these products around have become increasingly long, tortuous and diverse
Today’s multi-step cold-chains require the seamless integration of a large number of parties across several shipping modes, climate zones, storage facilities and patient health facilities.
And even when robust thermal management systems are in place and rigorously enforced, the problem is not necessarily solved. Simple human error or uncontrolled events such as a vial left out of a fridge or an unexpected power dropout, might spell the difference between life and death for an unfortunate patient further down the line.
Other circumstances where there can be life threatening breaks in the cold chain include the delivery of vaccines, clinical trial materials and other essential drugs into remote regions of the world where the infrasructure is often not available to ensure safe final-mile delivery.
THE REGULATORY POSITION
However it is important to note that regulatory authorities require that a manufacturer ensures product quality not only during storage and transport but until the drug concerned is used for patient treatment. This stipulation presents a formidable hurdle for a pharma manufacturer, since the conditions for product safety must be safeguarded even after the controls by the manufacturer in the primary supply chain have come to an end. This, for example, would include the transfer and storage of pharmaceutical products to and from pharmacists, retailers, hospitals and community doctors and even extends to when consumers take and store the product at home.
Official guidance in this respect is unequivocal and invariably recommends that drugs that are subjectively judged at point of use to be degraded or out of tolerance should be discarded. For example, the Centers for Disease Control and Prevention in the US advises: “It is better to not vaccinate than to administer a dose of vaccine that has been mishandled.”
DOMESTIC SAFETY
All of this begs the question: “How many bathroom cabinets and kitchen cupboards are full of pharma products that are of questionable quality?”
Today’s consumers are much too busy to read the fine print of every pharma label that comes their way. Few are competent to judge whether a product they buy or have stored for a time is safe or fit for purpose. Practically none can vouch for the temperature storage record of their household pharma supplies.
A recent study(1) provided the users of a temperaturesensitive biologic drug with a data recorder to monitor the real storage temperature conditions that were being used. The results were startling. Out of 255 participants in the study only 17 (6.7pc) had stored their medication within the recommended temperature range. Of those who did not, 24.3 per cent had stored their medication for more than 2 hours outside of the recommended range.