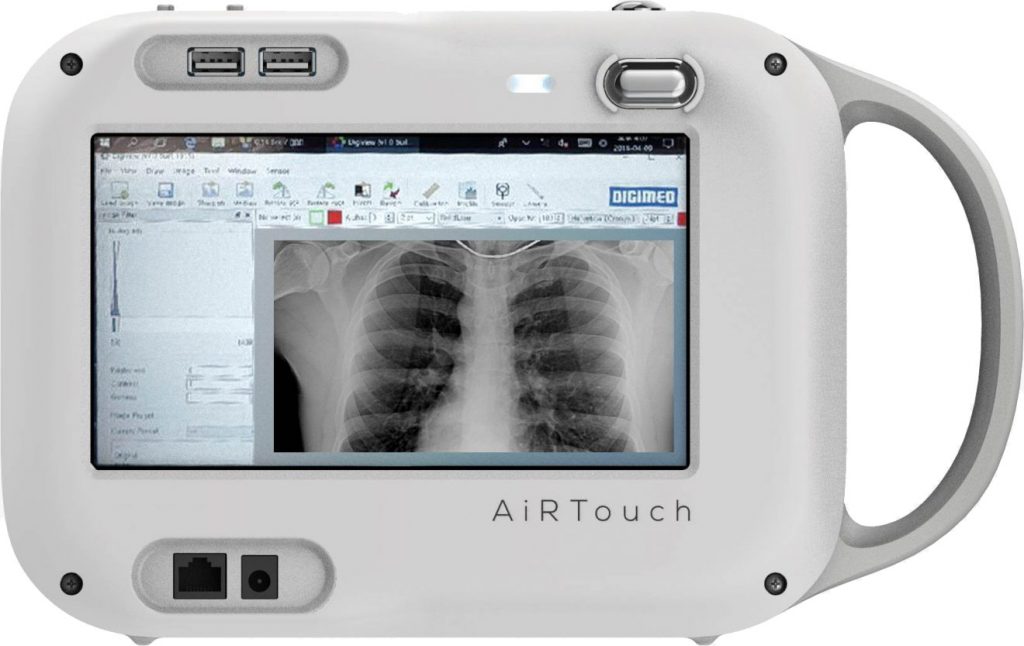
Aspenstate announced that it has received FDA clearance for the AiRTouch, a lightweight portable X-ray system that could be particularly useful for quickly obtaining chest X-rays of COVID-19 patients.
The handheld device weighs in at 5.5 pounds (2.5 Kg) and resembles a large digital camera with a touchscreen. AiRTouch acquires images with the push of a button and can wirelessly transmit them to PACS (clinical image storage system), without the need for a computer. Its battery charges within two hours and can capture up to 300 exposures per charge. Its portability has already made it useful in drive-through screening centers in South Korea, according to the company.
“Our clients have noticed a dramatic increase in capacity and the ability to move patients through quickly and efficiently,” David Lee, Vice President and COO of Aspenstate, said in a statement. “They find that the device is very simple to use, and the integrated software and portable features allow them to work outside of the limitations of traditional X-ray solutions.”
Besides using AiRTouch for chest X-rays for COVID-19, the company believes that it has a role in urgent care, ambulances, sports medicine, extremities, dental and veterinary settings.